This month on Blue Steens
It’s not an immunity passport
There seems to be a perception that a Covid-19 immunity passport is the same as a vaccination passport. This is not so. The choice of words matters!

Beyond Blue Steens
Coronavirus
⊳ Testing
🏴 We talk about PCR testing but don’t consider the ins and outs much. Here’s a Scottish firm that makes samples safe and, in doing so, streamlines testing.
⊳ Treatment
(Hydroxy-) Chloroquine:
The WHO has stopped its hydroxychloroquine and lopinavir/ritonavir (HIV drugs) trials in hospitalised patients. See March, May & June reviews for previous news. Both trials were discontinued due to insignificant reduction in mortality. Trials in non-hospitalised patients continue. However, lopinavir/ritonavir might cause heart problems.
Remdesivir:
USA has bought Gilead’s global stocks for July to Sep. If generics (see also June review) don’t come out quickly and their distribution isn’t widened, the rest of the world will barely get any supplies for the time being.
EMA has recommended conditional approval for treatment of severe cases.
Passive immunisation:
South Korea approves trials of Celltrion's neutralising antibody treatment (see also June review)
Last month I reported on cows producing human antibodies. This month I discovered that engineered llama antibodies have been found to neutralise the virus. As bizarre as this may sound, these are not the only animals whose antibodies have captured researchers’ attention. For example Elasmogen, an Aberdeen (Scotland) company, makes use of engineered antibody-like molecules inspired by sharks. They are also looking into developing COVID-19 applications. 🏴
Anti-inflammatory treatment:
Biocon’s Itolizumab has been approved in India at break-neck speed for emergency use against cytokine release syndrome.
Since the steroid drug dexamethasone appears to improve disease outcome (see also June review), the Infectious Diseases Society of America (IDSA) has now recommended and Japan has approved it as treatment.
It has been noted, however, that the treatment may not be as effective for patients over 70.
 #COVID19 This figure is probably the most interesting new info in #Dexamethasone NEJM paper nejm.org/doi/full/10.10… It appears the mortality benefit observed in RECOVERY trial was largely driven by age<70 pts. No benefit shown for pts >70yo, i.e. the most vulnerable population.
#COVID19 This figure is probably the most interesting new info in #Dexamethasone NEJM paper nejm.org/doi/full/10.10… It appears the mortality benefit observed in RECOVERY trial was largely driven by age<70 pts. No benefit shown for pts >70yo, i.e. the most vulnerable population.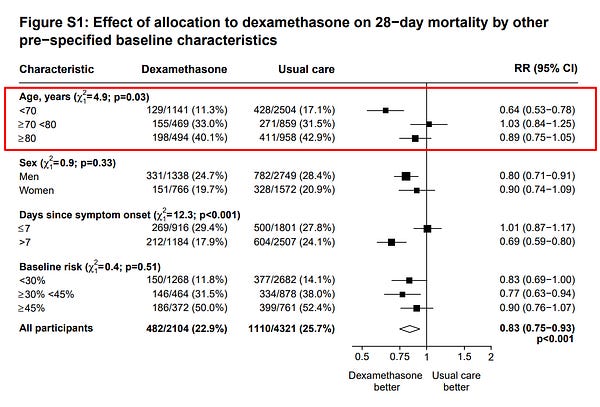
Other:
Inconclusive results from favipiravir (Avigan) Japan trial but promising results in India phase 3 trial (see also May review).
Novartis launches first-of-its-kind not-for-profit portfolio of medicines for symptomatic treatment. 15 generic and OTC medicines to be sold in up to 79 countries.
I try and steer clear of vague suggestions of potential treatments. However, EPO has caught my attention because it highlights something that’s fundamentally flawed in the prevailing drug development incentive system. There’s anecdotal evidence and academically founded hypotheses that EPO can improve the COVID-19 disease course. The problem is that there’s little incentive for pharma companies to get interested because there is no more patent protection.
FDA’s Coronavirus Treatment Acceleration Program (CTAP) website has some nice infographics and charts that track therapeutic developments.
⊳ Vaccination
India striving to launch its own vaccine by mid-August 2020. However, it is unclear whether the timeline is realistic.
Sinovac Biotech’s phase 3 trial in Brazil about to commence. With this progress, the company is hard on the heels of Sinopharm, AstraZeneca and Moderna.
Promising preliminary phase 1 trial data from Inovio. Phase 2/3 trial expected this summer.
Russia has two vaccines in clinical trials.
Biotech
Takeda & Carmine Therapeutics join forces developing and commercialising a novel non-viral rare disease gene therapy that uses a red blood cell extracellular vesicles (RBCEV) platform. RBCEV are thought to avoid side effects seen with the traditional virus-based approach, allow repeat-dosing, can carry more genetic material and be modified to improve distribution in the body.
EMA approval for Bristol-Myers Squibb’s first-in-class anaemia drug Reblozyl (luspatercept) for patients dependent on blood transfusion. It is an erythroid maturation agent that supports the patient’s body in producing its own red blood cells.
Out and about
I find myself in loads of online events these days, some of which kindly release their replays. In addition to hyperlinking these below, I have started compiling my favourites and other relevant videos I come across in a blockchain playlist on YouTube.
1 July: VC Investment into Blockchain- MMC Ventures on Why Blockchain and Why Now [replay]
2 July: A Framework for Blockchain Interoperability [replay]
2 July: Enterprise DLT Live Episode 003 - Luis Macias, GrainChain [replay]
9 July: Accelerating Clinical Trials by Leveraging Blockchain Technology Solutions [replay]
15 July: Book Talk Session 1: COVID-19 and Blockchain for Medical Research [replay]
16 July: Enterprise DLT Live Episode 004 - Darrell O'Donnell, CULedger [replay]
21 July: Acceleration of Remote Clinical Trials Using BlockChain [replay]
There’s a nice wee video about halfway through the session that demos the blockchain solution. Overall, I preferred the recent Health Unchained podcast with Rama Rao (CEO Bloqcube) on the topic though:
22 July: Reassessing life sciences supply chains to increase pandemic resilience and governmental actions [register for replay]
22 July: Book Talk Session 2: Blockchain for Medical Research [replay]
27 July: STOP COVID-19 Hackathon: What's Next? [will hyperlink if replay becomes available]
29 July: Pandemic Impact & Future of Blockchain [replay]
30 July: Episode 005 Enterprise DLT Live - Adam Krellenstein, Symbiont [replay]
Connect on Twitter, LinkedIn or YouTube if you like.
In this newsletter I share personal views and observations only. For more information and the privacy policy visit Blue Steens.


